Neurotech Pharmaceuticals, Inc., a private biotech company focused on developing therapies for chronic eye diseases, has announced the first commercial manufacturing, shipment, and surgical procedure for ENCELTO (revakinagene taroretcel-lwey), an encapsulated cell-based gene therapy and the first and only FDA-approved treatment for adults with idiopathic macular telangiectasia type 2 (MacTel).
“This is a huge milestone for people living with MacTel. For the first time, there is an FDA-approved treatment option that could change what is possible for patients. We have been working toward this for years, and seeing it become a reality is incredibly exciting for the Neurotech team and most importantly, for the patients who may benefit,” said Richard Small, CEO.
“Performing the first ENCELTO surgery for a patient with MacTel outside of a clinical trial is an important milestone. For the first time, we can offer a valuable and durable treatment option for patients, one that slows their loss of photoreceptors and maintains more visual function over time,” said Charles C. Wykoff, Retinal Consultants of Texas, Houston, TX.
“Many patients with MacTel experience gradual and progressive functional declines over time, and it is exciting to be able to offer an option to change the course of their disease.”
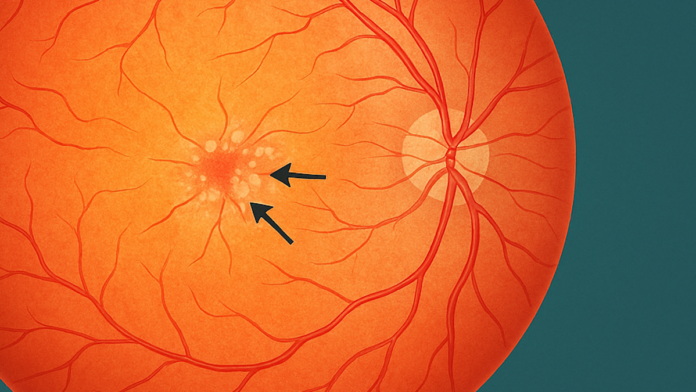
Macular telangiectasia type 2 (MacTel), or idiopathic macular telangiectasia type 2, is a bilateral, neurodegenerative disease in adults with characteristic localised retinal degeneration that causes the gradual loss of cells in the retina, resulting in vision loss and secondary alterations of the retinal vasculature, the network of blood vessels that supplies oxygen and nutrients to the retina.
ENCELTO is an encapsulated cell-based gene therapy designed to provide long-term, sustained delivery of therapeutic proteins for the treatment of chronic eye diseases. ENCELTO is the first technology to use Neurotech’s ECT platform, consisting of a small, semi-permeable capsule containing the proprietary allogeneic retinal pigment epithelium (RPE) cells genetically engineered to produce specific therapeutic proteins for targeted disease treatment.
The capsule is surgically inserted. Once in place, the capsule’s semi-permeable exterior membrane delivers the therapeutic proteins into the eye where they can travel to the retina located at the back of the eye. The exterior membrane protects the encapsulated RPE cells from the host’s immune system, contributing to their survival and functionality over time.