In the global battle against antimicrobial resistance (AMR), an international research team has unveiled what it says is a “powerful new game-changer.”
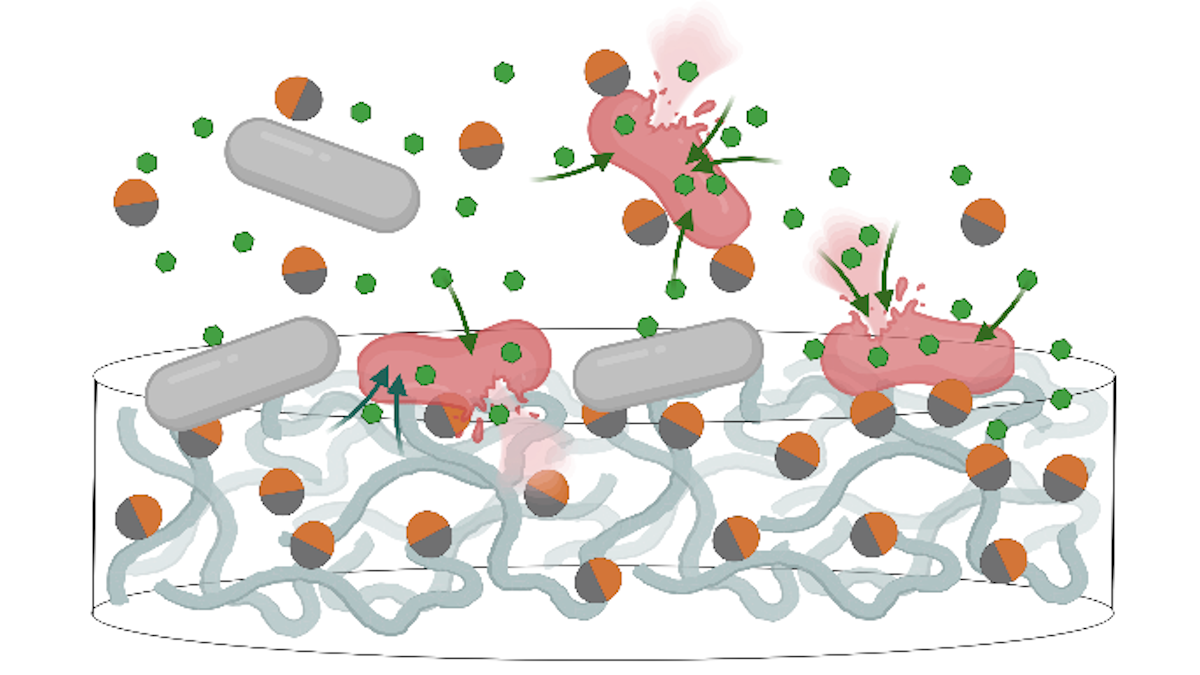
Using “two-faced” Janus nanoparticles, the team demonstrated a strategy that works in tandem with antibiotics to overcome the defences of highly resistant Gram-negative bacteria. These particles disrupt the bacteria’s protective outer membrane, creating an entry point for drugs that were previously ineffective, thereby reviving their life-saving potential.
AMR is a severe global health crisis, with drug-resistant Gram-negative bacteria posing a particular challenge. These microbes are encased in a strong outer membrane that acts as a shield, blocking many antibiotics from reaching their targets within the cell. This natural armour is a key reason why developing new drugs against them is so difficult and why existing ones are rapidly losing their power, leaving doctors with few treatment options.
Martijn Zwama and Kunihiko Nishino led the research at the Institute of Scientific and Industrial Research (SANKEN), The University of Osaka, in collaboration with Yan Yu (Indiana University; current position: Washington University in St. Louis, US), who led the design and engineering of the amphiphilic Janus nanoparticles (JNPs). Named after the two-faced Roman god, these particles have a dual nature: one side is hydrophilic (attracted to water) and the other is hydrophobic (repels water). This structure allows the JNPs to interact with and destabilize the bacterial outer membrane.
While the JNPs themselves are not lethal to the bacteria, they effectively create pores or disruptions on the surface, compromising the cell’s main defensive wall. This breach allows conventional antibiotic molecules, which were once blocked, to flood into the cell and execute their bacteria-killing function. This synergistic approach restored antibiotic activity against drug-resistant Gram-negative pathogens, including Escherichia coli and clinical isolates of Acinetobacter baumannii, a major cause of hospital-acquired infections.
This study offers a breakthrough materials-based strategy against AMR. By physically disrupting bacterial membranes, these nanoparticles are less susceptible to resistance evolution. They act as potent adjuvants, reviving and extending the lifespan of many existing antibiotics. This approach not only addresses a critical global health crisis but also opens doors for new clinical applications, such as antibacterial coatings, providing a tool to combat multidrug-resistant infections worldwide.
“These nanoparticles act as a perfect partner for antibiotics,” lead author Zwama said.
“They don’t kill the bacteria directly but rather open the door for antibiotics to do their job. This synergy overcame resistance in some of the most stubborn bacteria. This approach of ‘reviving’ antibiotics offers a promising and sustainable path forward in tackling the AMR crisis.”

