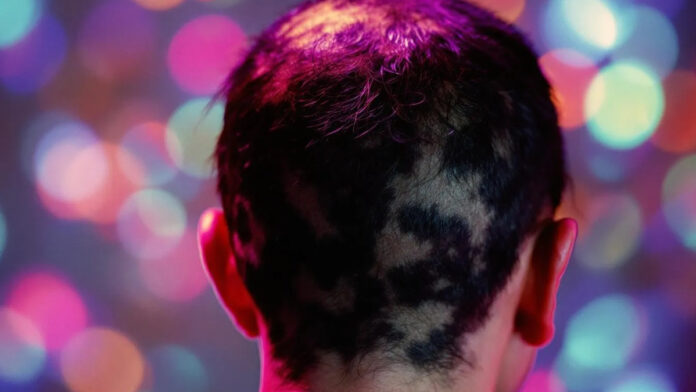
Nektar Therapeutics has announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for rezpegaldesleukin for the treatment of severe-to-very severe alopecia areata in adults and pediatric patients 12 years of age and older who weigh at least 40 kilogrammes.
Alopecia areata is a disease where a patient’s own immune system attacks hair follicles resulting in hair loss. The lifetime incidence of alopecia areata is 2% in both men and women. Nearly 6.7m people in the U.S. and 160m worldwide develop alopecia areata in their lifetime.
About 700,000 people in the US currently have some form of alopecia areata. It is often associated with other auto-immune conditions as well as depression and anxiety.
Rezpegaldesleukin is an investigational biologic therapy that targets the interleukin-2 receptor complex in the body to stimulate proliferation of inhibitory immune cells known as regulatory T cells (Tregs). Results from multiple clinical trials showed that rezpegaldesleukin safely and dose-dependently increased Tregs.
“We are pleased that rezpegaldesleukin has been granted Fast Track designation for the treatment of alopecia areata, adding to its Fast Track designation in atopic dermatitis,” said Jonathan Zalevsky, senior vice president and chief research and development officer at Nektar.
“Alopecia areata is a chronic, systemic, immune-mediated inflammatory disease, and there is an urgent need for novel mechanistic approaches that could treat the underlying pathogenesis of this disorder. We remain on track to announce topline data in December from our ongoing REZOLVE-AA phase 2b study for rezpegaldesleukin in alopecia areata, and we look forward to the opportunity to collaborate quickly with the agency on a potential registrational program following the completion of phase 2.”
Rezpegaldesleukin
Autoimmune and inflammatory diseases cause the immune system to mistakenly attack and damage healthy cells in a person’s body. A failure of the body’s self-tolerance mechanisms enables the formation of the pathogenic T lymphocytes that conduct this attack. Rezpegaldesleukin is a potential first-in-class resolution therapeutic that may address this underlying immune system imbalance in people with many autoimmune and inflammatory conditions. It targets the interleukin-2 receptor complex in the body to stimulate proliferation of powerful inhibitory immune cells known as regulatory T cells. By activating these cells, rezpegaldesleukin may act to bring the immune system back into balance.
Rezpegaldesleukin is being developed as a self-administered injection for autoimmune and inflammatory diseases. It is wholly owned by Nektar Therapeutics.
Jim Cornall is editor of Deeptech Digest and publisher at Ayr Coastal Media. He is an award-winning writer, editor, photographer, broadcaster, designer and author. Contact Jim here.