Ambros Therapeutics, Inc. has launched as a clinical-stage biotechnology company advancing late-stage therapies for severe, underserved diseases beginning with neridronate for complex regional pain syndrome type 1 (CRPS-1).
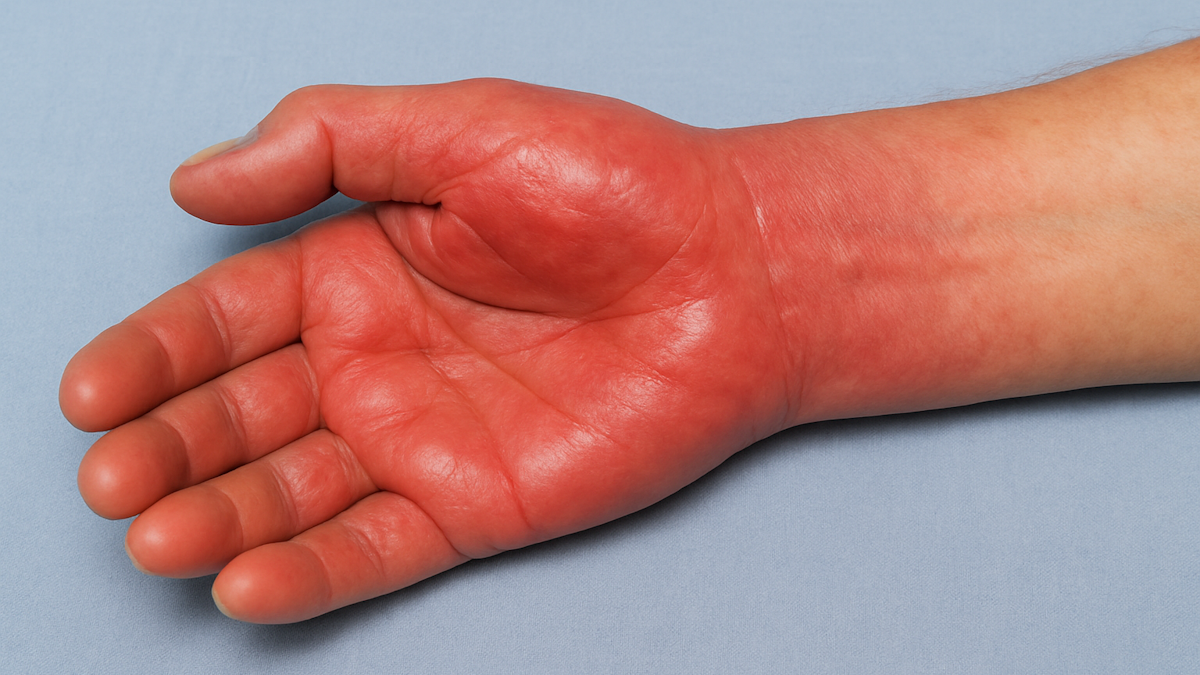
CRPS-1 is a rare and debilitating condition following an injury or trauma to the bone. There are currently no FDA-approved medicines available to treat this high unmet need patient population. The condition is characterized by intense pain that can be continuous in the affected limb – typically in extremities such as the arm, leg, hand or foot. Patients with CRPS-1 experience an evolving condition commencing with a “warm” phase lasting approximately six to 12 months after an initial injury where inflammation causes the affected limb to become red, swollen, warm, and painful to a chronic “cold” phase, where the affected limb changes its presentation but still faces ongoing, debilitating pain.
The company launched with an oversubscribed $125m Series A financing co-led by RA Capital Management and Patient Square Capital’s platform Enavate Sciences, joined by Abiogen Pharma, Janus Henderson Investors, Arkin Bio, Balyasny Asset Management, Transhuman Capital, Adage Capital Partners LP, and other dedicated life sciences investors.
The financing is expected to support the neridronate pivotal phase 3 clinical trial in CRPS-1 (CRPS-RISE) and related regulatory preparations and pre-commercial activities. Ambros licensed the rights to neridronate from the Italian pharmaceutical company, Abiogen Pharma S.p.A., under a strategic collaboration, providing Ambros with exclusive rights to neridronate in North America, with an option for broader market expansion.
Abiogen Pharma discovers and develops bisphosphonates including neridronate. Neridronate was approved in Italy based on the safety and efficacy demonstrated in two CRPS phase 3 studies and has been used for CRPS and other disorders in more than 600,000 patients to date.
The U.S. Food and Drug Administration (FDA) has granted neridronate Breakthrough Therapy, Fast Track, and Orphan Drug designations for the treatment of CRPS. CRPS-1, the subtype Ambros is targeting, is a severe, debilitating rare disease with an estimated 65,000 new cases annually in the US.
“We are launching Ambros with a seasoned leadership team and a therapy that has been used to treat CRPS in Italy for over 10 years,” said Jay Hagan, chief executive officer of Ambros Therapeutics.
“With no approved medicines for CRPS-1 outside of Italy, we look forward to working with Abiogen Pharma to advance neridronate through phase 3 and bring this therapy to patients who urgently need it.”
“We are excited to partner with Ambros to bring neridronate to patients outside of Italy, where there are currently no approved medicines,” said Massimo Di Martino, president of Abiogen Pharma.
“With decades of leadership in bisphosphonate innovation at our dedicated research and development centre, we believe in the potential for neridronate to address CRPS outside of Abiogen’s core commercial markets.”